The global lentiviral vectors market is expected to grow from USD 368.3 million in 2019 to USD 1.08 billion by 2027, at a CAGR of 14.52% during the forecast period 2020-2027.
Lentiviral vectors are utilized as a method to deliver extraneous genetic material into another cell. The vectors are generated from lentivirus, a type of retrovirus identified by its lengthy incubation period and can affect both non-dividing cells and dividing cells. The lentiviral vectors are originated from human immunodeficiency virus (HIV), and therefore, these vectors are highly effective vehicles for in vivo gene transmission for gene therapies. Lentiviruses are utilized for transfection in actively dividing and non-dividing cells. It serves as an advantage to a different type of retrovirus. The lentiviruses come in the category of a retrovirus. The lentiviral vector system is formed, which can alter the gene of interest. The commercially available lentiviral vector systems are available in combination with the reporter gene, promoters, tag sequence, and multicistronic vectors available for desired protein composition. These vectors are utilized in order to research and develop the purpose of protein characterization, in-vitro transcription, recombinant protein expression, and other functional arrays.
This study delivers a comprehensive analysis of diseases, workflows and regions. The disease segment includes infectious diseases, genetic disorders and cancer. The genetic disorder segment is expected to show the highest growth in the lentiviral vectors market over the forecast period. Currently, about two-thirds of the examination in gene therapeutics is concentrated on oncology. Rare and hereditary illnesses are added to vital areas of importance in gene therapeutics. It is because approximately 350 million patients are diagnosed globally with a rare disease, and there is a shortage of efficient remedy modalities. The increasing burden of genetic disorders is a significant driver for investment in viral vector production to target genetic disease. The workflow segment includes downstream processing and upstream processing. The growing need for vectors to meet the increasing demand for robust therapies has pronounced the need to optimize downstream processing workflows and upstream processing. Virus preparation designs at small-scale include steps that are hard to scale-up and are usually considered tedious. It has occurred in the optimization and the investigation of different scalable business methods to ensure the number of viruses while preserving their significant quality properties. Downstream processing is estimated to account for the higher share over the forecast period due to extremely complex processes carried out for the purification and polishing of clinical-grade final outputs. Further, the high cost of purifying procedures has driven to high revenue creation in this segment.
The market has been divided into North America, Europe, Asia-Pacific, Middle East & Africa, and South America. Industrialization and clinical transformation of gene therapeutics production are anticipated to influence notable progress over the forecast period across Asian countries. The global corporations are collaborating with Asia-based businesses to stimulate vector production in Asian countries. In March 2019, Merck approved a Memorandum of Understanding with China-based GenScript, under which Merck proposed to give GenScript with extensive services, products, and training on viral vector manufacturing. With such collaboration, Asian companies intended to offer improved cGMP production capacities to their local and overseas clients.
Some of the notable players in the global lentiviral vectors market areSirion-Biotech GmbH, OriGene Technologies, Sino Biological Inc., Cell Biolabs, Inc., bluebird bio, Inc., Thermo Fisher Scientific, SignaGen Laboratories, GenTarget Inc., Vigene Biosciences, GENEMEDI and Takara Bio Inc.
This study forecasts revenue growth at global, regional, and country levels from 2020 to 2027. Fior Markets has segmented the market based on the below-mentioned segments:
Global Lentiviral Vectors Market Analysis And Forecast, By Disease
Global Lentiviral Vectors Market Analysis And Forecast, By Workflow
Global Lentiviral Vectors Market Analysis And Forecast, By Regional Analysis
Report Description:
1. Introduction
1.1. Objectives of the Study
1.2. Market Definition
1.3. Research Scope
1.4. Currency
1.5. Key Target Audience
2. Research Methodology and Assumptions
3. Executive Summary
4. Premium Insights
4.1. Porter’s Five Forces Analysis
4.2. Value Chain Analysis
4.3. Top Investment Pockets
4.3.1. Market Attractiveness Analysis By Disease
4.3.2. Market Attractiveness Analysis By Workflow
4.3.3. Market Attractiveness Analysis By Region
4.4. Industry Trends
5. Market Dynamics
5.1. Market Evaluation
5.2. Drivers
5.2.1. Production of cost-effective and accelerated protein composition methods
5.3. Opportunities
5.3.1. Private and government-funded initiatives which concentrate on research and development programs
5.4. Restraints
5.4.1. High cost of manufacturing and gene therapies
6. Global Lentiviral Vectors Market Analysis and Forecast, By Disease
6.1. Segment Overview
6.2. Infectious Diseases
6.3. Genetic Disorders
6.4. Cancer
7. Global Lentiviral Vectors Market Analysis and Forecast, By Workflow
7.1. Segment Overview
7.2. Downstream Processing
7.2.1. Fill-finish
7.2.2. Purification
7.3. Upstream Processing
7.3.1. Vector Recovery/Harvesting
7.3.2. Vector Amplification & Expansion
8. Global Lentiviral Vectors Market Analysis and Forecast, By Regional Analysis
8.1. Segment Overview
8.2. North America
8.2.1. U.S.
8.2.2. Canada
8.2.3. Mexico
8.3. Europe
8.3.1. Germany
8.3.2. France
8.3.3. U.K.
8.3.4. Italy
8.3.5. Spain
8.4. Asia-Pacific
8.4.1. Japan
8.4.2. China
8.4.3. India
8.5. South America
8.5.1. Brazil
8.6. Middle East and Africa
8.6.1. UAE
8.6.2. South Africa
9. Global Lentiviral Vectors Market-Competitive Landscape
9.1. Overview
9.2. Market Share of Key Players in Global Lentiviral Vectors Market
9.2.1. Global Company Market Share
9.2.2. North America Company Market Share
9.2.3. Europe Company Market Share
9.2.4. APAC Company Market Share
9.3. Competitive Situations and Trends
9.3.1. Workflow Launches and Developments
9.3.2. Partnerships, Collaborations, and Agreements
9.3.3. Mergers & Acquisitions
9.3.4. Expansions
10. Company Profiles
10.1. Sirion-Biotech GmbH
10.1.1. Business Overview
10.1.2. Company Snapshot
10.1.3. Company Market Share Analysis
10.1.4. Company Disease Portfolio
10.1.5. Recent Developments
10.1.6. SWOT Analysis
10.2. OriGene Technologies
10.2.1. Business Overview
10.2.2. Company Snapshot
10.2.3. Company Market Share Analysis
10.2.4. Company Disease Portfolio
10.2.5. Recent Developments
10.2.6. SWOT Analysis
10.3. Sino Biological Inc.
10.3.1. Business Overview
10.3.2. Company Snapshot
10.3.3. Company Market Share Analysis
10.3.4. Company Disease Portfolio
10.3.5. Recent Developments
10.3.6. SWOT Analysis
10.4. Cell Biolabs, Inc.
10.4.1. Business Overview
10.4.2. Company Snapshot
10.4.3. Company Market Share Analysis
10.4.4. Company Disease Portfolio
10.4.5. Recent Developments
10.4.6. SWOT Analysis
10.5. bluebird bio, Inc.
10.5.1. Business Overview
10.5.2. Company Snapshot
10.5.3. Company Market Share Analysis
10.5.4. Company Disease Portfolio
10.5.5. Recent Developments
10.5.6. SWOT Analysis
10.6. Thermo Fisher Scientific
10.6.1. Business Overview
10.6.2. Company Snapshot
10.6.3. Company Market Share Analysis
10.6.4. Company Disease Portfolio
10.6.5. Recent Developments
10.6.6. SWOT Analysis
10.7. SignaGen Laboratories
10.7.1. Business Overview
10.7.2. Company Snapshot
10.7.3. Company Market Share Analysis
10.7.4. Company Disease Portfolio
10.7.5. Recent Developments
10.7.6. SWOT Analysis
10.8. GenTarget Inc.
10.8.1. Business Overview
10.8.2. Company Snapshot
10.8.3. Company Market Share Analysis
10.8.4. Company Disease Portfolio
10.8.5. Recent Developments
10.8.6. SWOT Analysis
10.9. Vigene Biosciences
10.9.1. Business Overview
10.9.2. Company Snapshot
10.9.3. Company Market Share Analysis
10.9.4. Company Disease Portfolio
10.9.5. Recent Developments
10.9.6. SWOT Analysis
10.10. GENEMEDI
10.10.1. Business Overview
10.10.2. Company Snapshot
10.10.3. Company Market Share Analysis
10.10.4. Company Disease Portfolio
10.10.5. Recent Developments
10.10.6. SWOT Analysis
10.11. Takara Bio Inc.
10.11.1. Business Overview
10.11.2. Company Snapshot
10.11.3. Company Market Share Analysis
10.11.4. Company Disease Portfolio
10.11.5. Recent Developments
10.11.6. SWOT Analysis
List of Table
1. Global Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
2. GlobalInfectious Diseases, Lentiviral Vectors Market, By Region, 2017-2027 (USD Billion) (K Units)
3. Global Genetic Disorders, Lentiviral Vectors Market, By Region, 2017-2027 (USD Billion) (K Units)
4. Global Cancer, Lentiviral Vectors Market, By Region, 2017-2027 (USD Billion) (K Units)
5. Global Lentiviral Vectors Market, ByWorkflow, 2017-2027 (USD Billion) (K Units)
6. GlobalDownstream Processing, Lentiviral Vectors Market, By Region, 2017-2027 (USD Billion) (K Units)
7. Global Upstream Processing, Lentiviral Vectors Market, By Region, 2017-2027 (USD Billion) (K Units)
8. North America Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
9. North America Lentiviral Vectors Market, ByWorkflow, 2017-2027 (USD Billion) (K Units)
10. U.S. Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
11. U.S. Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
12. Canada Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
13. Canada Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
14. Mexico Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
15. Mexico Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
16. Europe Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
17. Europe Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
18. Germany Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
19. Germany Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
20. France Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
21. France Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
22. U.K. Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
23. U.K. Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
24. Italy Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
25. Italy Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
26. Spain Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
27. Spain Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
28. Asia Pacific Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
29. Asia Pacific Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
30. Japan Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
31. Japan Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
32. China Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
33. China Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
34. India Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
35. India Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
36. South America Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
37. South America Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
38. Brazil Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
39. Brazil Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
40. Middle East and Africa Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
41. Middle East and Africa Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
42. UAE Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
43. UAE Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
44. South Africa Lentiviral Vectors Market, By Disease, 2017-2027 (USD Billion) (K Units)
45. South Africa Lentiviral Vectors Market, By Workflow, 2017-2027 (USD Billion) (K Units)
List of Figures
1. Global Lentiviral Vectors Market Segmentation
2. Global Lentiviral Vectors Market: Research Methodology
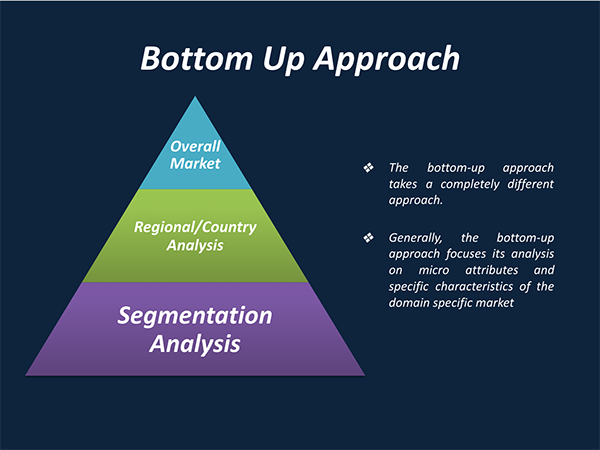
3. Market Size Estimation Methodology: Bottom-Up Approach
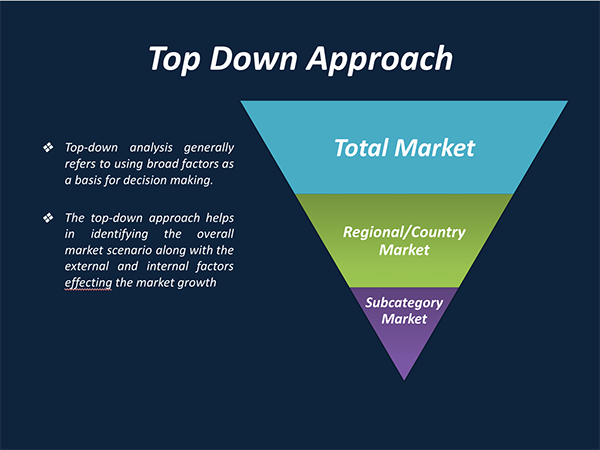
4. Market Size Estimation Methodology: Top-Down Approach
5. Data Triangulation
6. Porter’s Five Forces Analysis
7. Value Chain Analysis
8. Global Lentiviral Vectors Market Attractiveness Analysis by Disease
9. Global Lentiviral Vectors Market Attractiveness Analysis by Workflow
10. Global Lentiviral Vectors MarketAttractiveness Analysis by Region
11. Global Lentiviral Vectors Market: Dynamics
12. Global Lentiviral Vectors Market Share by Disease(2020 & 2027)
13. Global Lentiviral Vectors Market Share by Workflow (2020 & 2027)
14. Global Lentiviral Vectors Market Share by Regions (2020 & 2027)
15. Global Lentiviral Vectors Market Share by Company (2019)
Market research is a method of gathering, assessing and deducing data & information about a particular market. Market research is very crucial in these days. The techniques analyze about how a product/service can be offered to the market to its end-customers, observe the impact of that product/service based on the past customer experiences, and cater their needs and demands. Owing to the successful business ventures, accurate, relevant and thorough information is the base for all the organizations because market research report/study offers specific market related data & information about the industry growth prospects, perspective of the existing customers, and the overall market scenario prevailed in past, ongoing present and developing future. It allows the stakeholders and investors to determine the probability of a business before committing substantial resources to the venture. Market research helps in solving the marketing issues challenges that a business will most likely face.
Market research is valuable because of the following reasons:
Our research report features both the aspects; qualitative and quantitative. Qualitative part provides insights about the market driving forces, potential opportunities, customer’s demands and requirement which in turn help the companies to come up with new strategies in order to survive in the long run competition. The quantitative segment offers the most credible information related to the industry. Based on the data gathering, we use to derive the market size and estimate their future growth prospects on the basis of global, region and country.
Our market research process involves with the four specific stages.
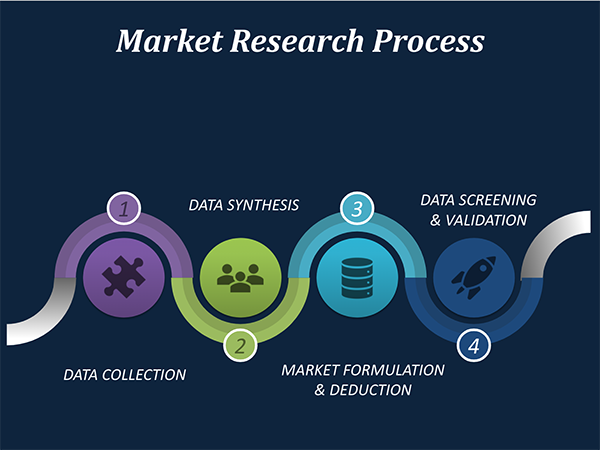
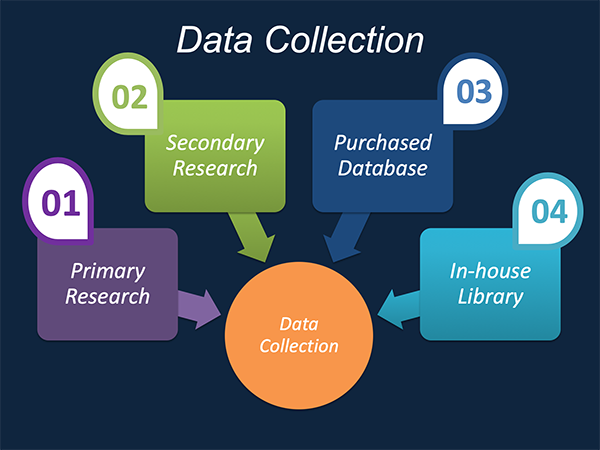
Data Collection: This stage of the market research process involves with the gathering and collecting of the market/industry related data from the sources. There are basically two types of research methods:
Data Synthesis: This stage includes the evaluation and assessment of all the data acquired from the primary and secondary research. It likewise includes in evaluating the information for any disparity watched while information gathering identified with the market. The data & information is gathered with consideration to the heterogeneity of sources. Scientific and statistical methods are implemented for synthesizing dissimilar information sets and provide the relevant data which is fundamental for formulating strategies. Our organization has broad involvement with information amalgamation where the information goes through different stages:
Market Formulation & Deduction: The last stage includes assigning the data & information in a suitable way in order to derive market size. Analyst reviews and domain based opinions based on holistic approach of market estimation combined with industry investigation additionally features a crucial role in this stage.
This stage includes with the finalization of the market size and numbers that we have gathered from primary and secondary research. With the data & information addition, we ensure that there is no gap in the market information. Market trend analysis is finished by our analysts by utilizing data extrapolation procedures, which give the most ideal figures to the market.
Data Validation: Validation is the most crucial step in the process. Validation & re-validation through scientifically designed technique and process that helps us finalize data-points to be used for final calculations. This stage also involves with the data triangulation process. Data triangulation generally implicates the cross validation and matching the data which has been collected from primary and secondary research methods.













































Free Customization
Countries can be added on demand
Free yearly update on purchase of Multi/Corporate User License
Companies served till date

We serve our customers 24x7 for 365 days through calls, emails and live chat options.
Huge database of exceptional market reports bringing market intelligence to your fingertips.

SSL enabled, we offer you various secured payment options for risk free purchase.